mainmenu
Research
Prof.Kisuk Kang
Synthesis and application of nanocrystals
1Energy material design : First principles Calculation
A calculation is said to be from first principles, or ab initio, if it starts directly at the level of established laws of physics and does not make assumptions such as model and fitting parameters. We have performed first principles calculations using state-of-the-art DFT (Density Functional Theory) for energy material design.
Solar cells have many applications. Cells are used for powering small devices such as electronic calculators. Photovoltaic arrays generate a form of renewable electricity, particularly useful in situations where electrical power from the grid is unavailable such as in remote area power systems, Earth-orbiting satellites and space probes, remote radiotelephones and water pumping applications. Photovoltaic electricity is also increasingly deployed in grid-tied electrical systems. Similar devices intended to capture energy from other sources include thermophotovoltaic cells, betavoltaics cells, and optoelectric nuclear batteries.
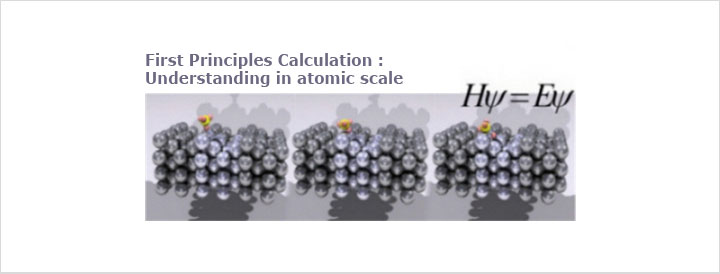
2Lithium Ion Batteries
Lithium-ion batteries are a type of rechargeable battery in which a lithium ion moves between the anode and cathode. The lithium ion moves from the anode to the cathode during discharge and from the cathode to the anode when charging.
Lithium ion batteries are commonly used in consumer electronics. They are currently one of the most popular types of battery for portable electronics, with one of the best energy-to-weight ratios, no memory effect, and a slow loss of charge when not in use. In addition to uses for consumer electronics, lithium-ion batteries are growing in popularity for defense, automotive, and aerospace applications due to their high energy density. However certain kinds of mistreatment may cause Li-ion batteries to explode.
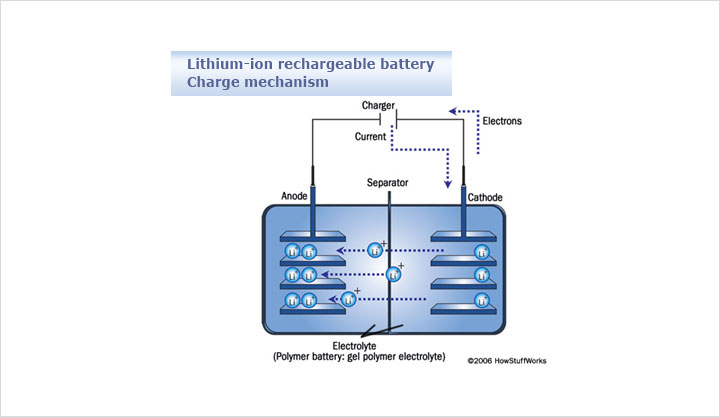
3Hydrogen Storage Materials
The traditional hydrides have excellent H-volume storage capacity, good and tunable kinetics and reversibility, but poor H-storage by weight. Carbon nanotubes are now considered not to have any potential. On the other hand, highly porous carbon and hybrid materials have capability of high mass storage capacity, but since adsorption is of molecular hydrogen, they can only work at cryogenic conditions.
The light metal alloys have the required mass density, but poor kinetics and high absorption temperatures/pressures. The complex hydrides undergo chemical reactions while desorbing/adsorbing, thus restricting kinetics and reversibility. Hence, research on adequate H-storage materials remains a challenge in particular for the transportation sector.
4Fuel Cell
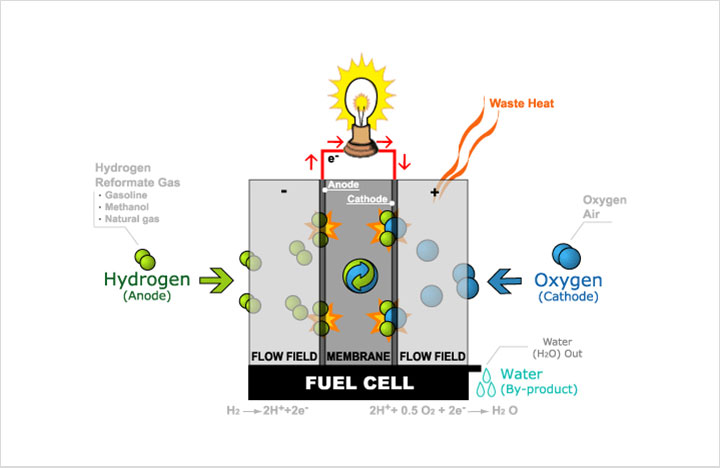
A fuel cell is an electrochemical conversion device. It produces electricity from fuel (on the anode side) and an oxidant (on the cathode side), which react in the presence of an electrolyte. The reactants flow into the cell, and the reaction products flow out of it, while the electrolyte remains within it. Fuel cells can operate virtually continuously as long as the necessary flows are maintained.
Fuel cells are different from electrochemical cell batteries in that they consume reactant, which must be replenished, whereas batteries store electrical energy chemically in a closed system. Additionally, while the electrodes within a battery react and change as a battery is charged or discharged, a fuel cell's electrodes are catalytic and relatively stable.
5Solar Cell
Solar cell is a large area electronic device that converts solar energy into electricity by the photovoltaic effect. Photovoltaics is the field of technology and research related to the application of solar cells for solar energy. Sometimes the term solar cell is reserved for devices intended specifically to capture energy from sunlight, while the term photovoltaic cell is used when the source is unspecified. Assemblies of cells are used to make solar modules, or photovoltaic arrays.