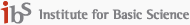mainmenu
Research
Prof.Yungeun Sung
Synthesis and application of nanocrystals
Prof. Sung’s group (Photo and electrochemical energy laboratory) belongs to the school of chemical and biological engineering at Seoul national university. Prof. Sung has led various electrochemical energy related projects, including the Center for materials of advanced batteries established in 2005, which ran for 5 years under the support from Ministry of Knowledge Economy of Korea.
Currently, there are 2 staff member, 2 post docs, 19 PhD students and 10 master students. Main studies include the electrochemical analyses of novel fuel cell catalysts and dye sensitized solar cell electrodes, and the analyses of the structure and the interface between electrode and electrolyte in lithium ion battery system. In addition, the group focuses on the design and the development of non-metal catalysts for fuel cell and novel electrode materials for advanced battery system, which encompass lithium sulfur battery and sodium ion battery.
1Solar Cell
Our research is focused on the development of efficient materials for solar cell application. Most of all, we have an interest in nano-structured materials for DSSC. Nano-structured TiO2 is a key material for efficient DSSC. We have researched about one dimensional TiO2 nanomaterials, i.e. TiO2 nanorod and nanotube. We have demonstrated that one dimensional TiO2 nanomaterials represent an enhanced charge transport characteristics when comparing with TiO2 nanoparticles. TiO2 nanorod can transport photoelectrons more efficiently than nanoparticle, due to the reduced grain boundaries which act as a recombination center. And, we also have a technology of preparing a vertically oriented TiO2 nanotube by using an anodization method. Because of its vertically oriented structure, it has advantages in light scattering and charge transport. These researches about nanomaterials will be able to enhance the cell efficiency of DSSC highly.
2Lithium ion Battery
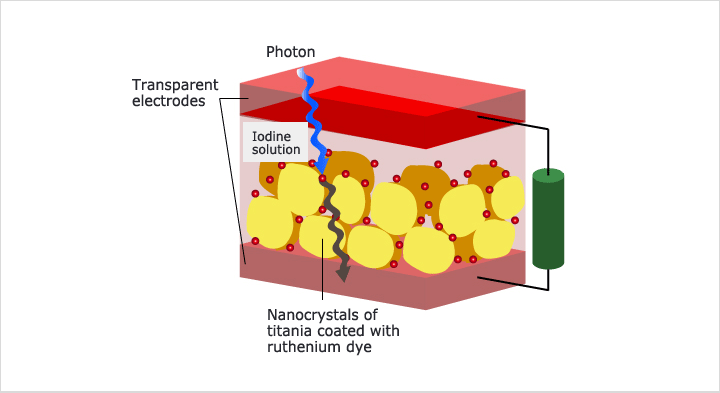
Our research efforts are focused on several aspects of the Li-ion battery including nanostructured electrode materials, analysis and hybrid solar-battery. Increasing life cycle and performance (decreasing internal resistance and increasing output power) by changing the composition of the material used in the anode and cathode along with increasing the effective surface area of the electrodes. Improving capacity by improving the structure to incorporate more active materials. Improving the safety of Lithium Ion style batteries. & analysis (XRD, Raman, IR, XPS) of electrochemical reaction mechanism.
3Fuel Cell
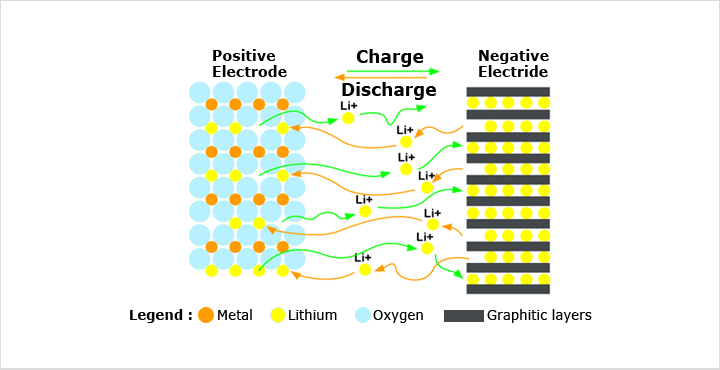
The fuel cell is similar to a battery. It produces electricity using chemicals. The chemicals are usually very simple, often just hydrogen and oxygen. In this case the hydrogen is the "fuel" that the fuel cell uses to make electricity. The very important difference between fuel cell and battery is that fuel cells do not run down like batteries. As long as the fuel and oxygen is supplied to the cell it will keep producing electricity forever. The oxygen needed by a fuel cell is usually simply obtained from air. Although the majority of fuel cells use hydrogen as the fuel, some fuel cells work off methane, and a few use liquid fuels such as methanol. Fuel cells that use hydrogen can be thought of as devices that do the reverse of the well known experiment where passing an electric current through water splits it up into hydrogen and oxygen. In the fuel cell, hydrogen and oxygen are joined together to produce water and electricity. Fuel cells can be made in a huge range of sizes. They can be used to produce quite small amounts of electric power, for devices such as portable computers or radio transmitters, right up to very high powers for electric power stations.
4Electrochromic Device
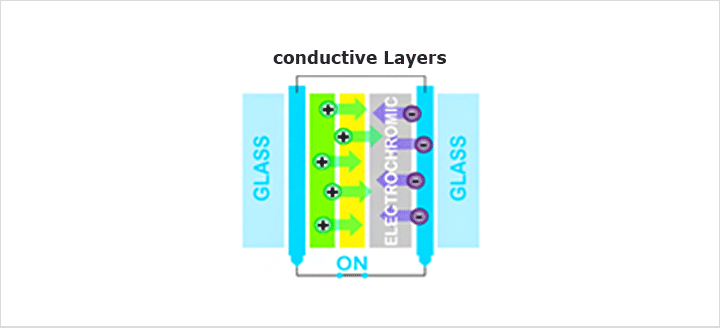
Our research is focused on the EC mirror and smart window fabricated by inorganic materials. In EC mirror, if intensive light comes, electrochromic materials go to the colored state and reduce the light by absorbing it. This system is applied for rear view mirrors in cars or trucks to prevent the dazzling during driving a car. Secondly, smart windows, the most important application of electrochromism, are used in energy-efficient architecture because they would adjust the inflow of luminous radiation and solar energy through glazing in buildings. Therefore, buildings save more energy in heating or air conditioning. We fabricate thin films of electrochromic material by many methods; RF sputtering method, DC sputtering method, sol-gel method, electrodeposition method, etc. Structures of these nano-scaled or micro-scaled thin films are required for porous structure to intercalate ion easily. Using these thin films, we assemble the EC devices, all solid state system and solid-liquid system, and measure electrochemical properties for testing EC performance. We aim to improve durability, stability and switch times required for color change.